Abstract
Introduction:
ENESTPath, an ongoing phase 3 study in patients with chronic myeloid leukemia (CML) in chronic phase, was designed to determine the optimal duration of consolidation treatment with nilotinib 300 mg twice daily required to remain in treatment-free remission (TFR) without molecular relapse, 12 months after entering TFR. With TFR as a new goal of therapy in clinical practice, there is a need for individual approaches and counselling for patients when preparing for a possible treatment discontinuation.
An ongoing Italian substudy of ENESTPath assesses patients' emotional experience during and after stopping nilotinib therapy. To have a comprehensive evaluation of patients' psycho-emotional outcomes, both quantitative (questionnaires) and qualitative (expressive writing and interviews) methods are used. Here, we present preliminary qualitative data on the emotional experience of patients who were either ineligible for randomization or relapsed during TFR phase.
Methods:
Patients in stable MR4 for >12 months (≥MR4 in 4 of 5 PCR assessments, including the last assessment, in the last 12 months) in the consolidation phase were randomized to stop nilotinib and enter TFR phase immediately or to continue on nilotinib for another year and then enter TFR phase. Patients with unstable MR4 in the consolidation phase had to discontinue the trial early without attempting randomization. Patients who were ineligible for randomization or those who relapsed during TFR phase (loss of MMR, or the confirmed loss of MR4) had qualitative interviews.
In-depth unstructured qualitative interviews were conducted according to interpretative phenomenological analysis (IPA) to explore patients' emotional experience of "being ineligible for randomization" and "failing TFR." Interviews were guided using open-ended questions and followed patients' narrative flow; the interviewer facilitated the narration. Patients were asked to describe the course of their disease and their involvement in the clinical trial, including the final moments before trial discontinuation. Interviews were audio recorded, transcribed verbatim, and analyzed according to IPA.
Results:
Of the 30 patients included in this substudy so far, 10 (male, n=8; mean disease duration, 9.5 years) were interviewed, including 7 ineligible for randomization and 3 relapsing in TFR. As the study is still ongoing, these numbers are not representative of the total study population. The remaining 20 patients were either in TFR, year 2 of consolidation, or yet to be interviewed. Enrollment for qualitative research respects the criterion of the most possible variety (eg, socio-demographic characteristics, duration of the disease).
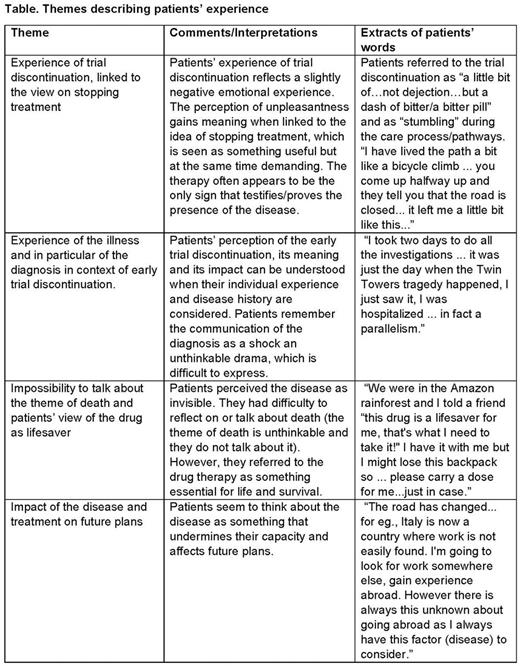
In total, four themes that describe patients' experience were identified: experience of early trial discontinuation for unstable response, linked to the view on stopping treatment; experience of the illness particularly focused on diagnosis; impossibility to talk about death and view on the drug as lifesaver; and impact of the disease and treatment on future plans (Table).
Patients' experience of not being able to continue the trial due to unstable response reflected a slightly negative emotional experience, and they saw the idea of stopping treatment as something useful but demanding. Patients remembered the communication of the diagnosis as an unthinkable drama that is difficult to express and a shock. They perceived the disease as invisible and had difficulties in thinking or talking about death; however, they referred to the drug therapy as essential for life and survival. Finally, the disease was also perceived as something that undermines future plans (Table).
Conclusions:
These preliminary findings described how patients perceived early trial discontinuation, and the way they give sense to it appears to have a relationship with individual disease experience. This qualitative analysis helps in understanding patients' perspective, both in terms of getting access to the inner subjective experience of having CML and its strict relationship with the involvement in a trial or its cessation. The insights provided show that patients' perception of their disease, on TFR, and on failure to stop the therapy needs further analysis. There is a need to develop strategies to cope with patients' emotional experience and guarantee their psychoemotional wellbeing beyond the clinical setting.
Castagnetti: Bristol Myers Squibb: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Bonifacio: Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cavazzini: Bristol-Meyers Squibb: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Incyte: Consultancy. Galimberti: Incyte: Speakers Bureau; Novartis: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Pfizer: Speakers Bureau. Orlandi: Incyte: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Novartis: Speakers Bureau. Galimberti: Novartis: Employment. Haenig: Novartis: Employment. Supekar: Novartis: Employment. Baccarani: Novartis: Consultancy, Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Speakers Bureau; Incyte ARIAD: Consultancy, Honoraria, Speakers Bureau. Vegni: Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal